In accordance with the requirements of the current Good Practices, we audit your suppliers in connection with your Drugs quality.
We audit your suppliers or service providers related to the quality of your medicines: Manufacturers and distributors of raw materials for pharmaceutical, cosmetic and alimentary purposes, manufacturers, subcontractors-analytical, depositories, carriers. Our customers are primarily human-sized groups, operators and / or manufacturers based in France or Europe (Switzerland, Belgium, Italy).
We carry out your audits in France, in Europe, and in the rest of the world.
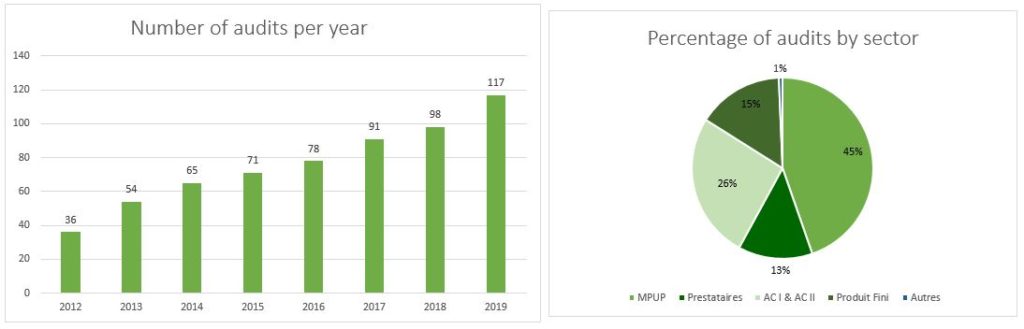
In 2019, we delivered 150 audit files. We audit according to the applicable specific quality standards (BPx and ISO).